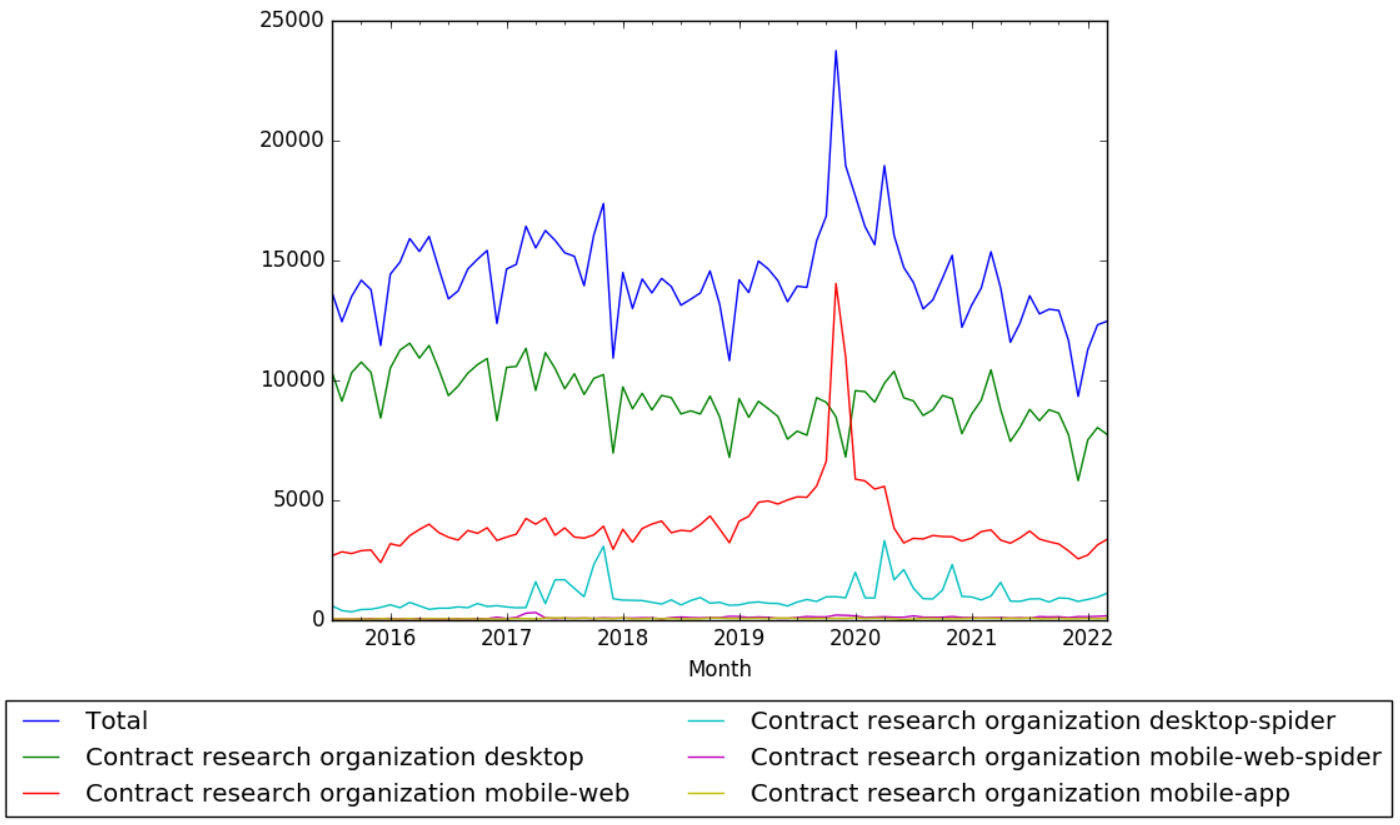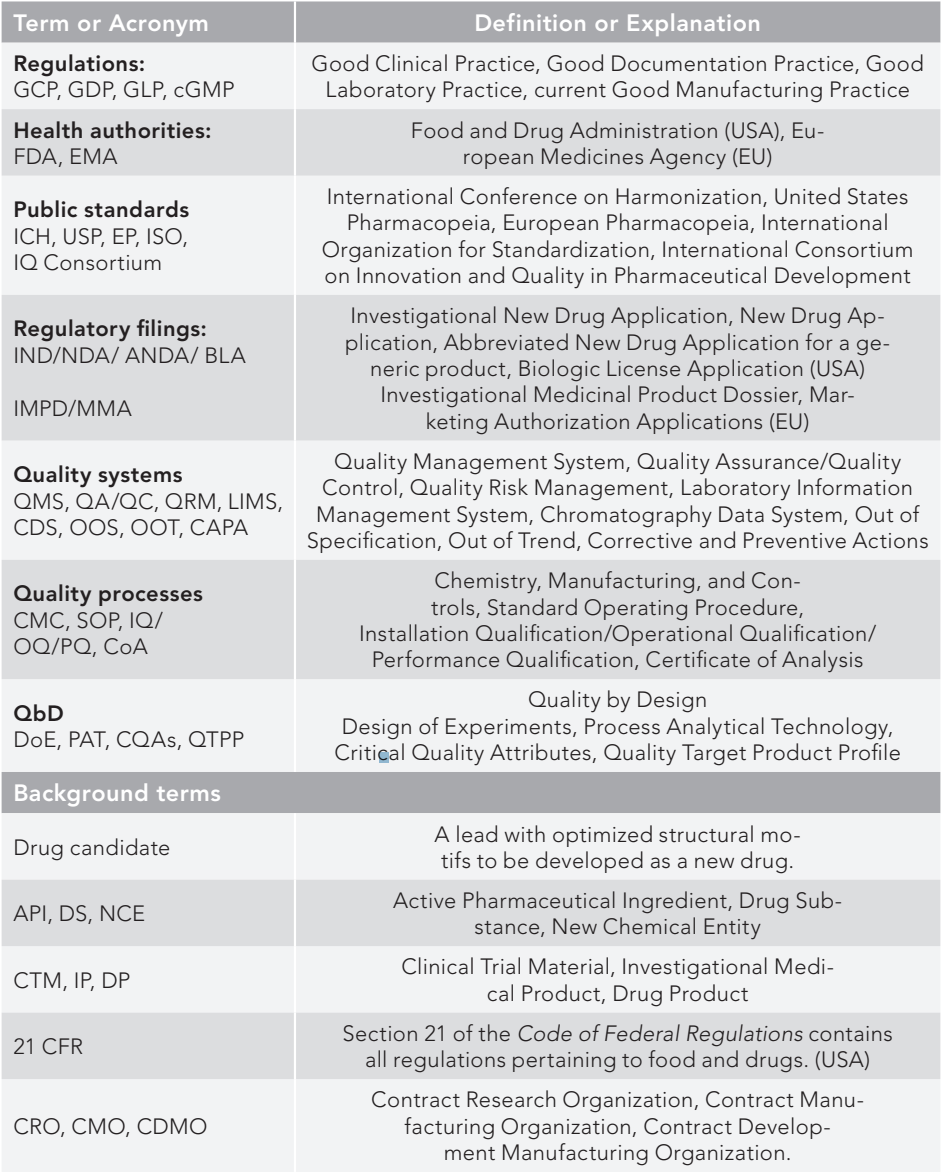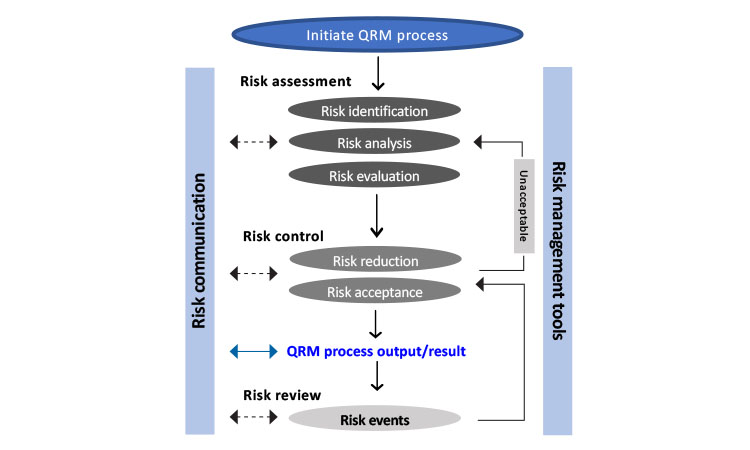
Clinical Trial Management Adaptation to ICH E6 (R2): Good Clinical Practice | Pharmaceutical Engineering

EU clinical research framework. ICH GCP = International Conference on... | Download Scientific Diagram

Introduction to Good Clinical Practice (GCP) Guidelines: Ensuring Quality in Clinical Research | PPT