THE DISSOCIATION CONSTANT OF ACETIC ACID FROM 0 TO 35° CENTIGRADE1 | Journal of the American Chemical Society
Illustrated Glossary of Organic Chemistry - Acid ionization constant (acid dissociation constant; Ka)
The pH of an acetic acid solution is 3.26. What is the concentration of acetic acid and what is the percent of acid that's ionized? - Quora

What is the pH of a 10 mM solution of acetic acid (CH3COOH)? Acetic Acid Ka= 1.76 x 10^{-5} M. | Homework.Study.com
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://i.ytimg.com/vi/AufT6_CoFWY/maxresdefault.jpg)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]

A 0 05 n sol of acetic acid is found to be 1 9+ ionised at 25 c calculate - Chemistry - Equilibrium - 13276973 | Meritnation.com
How to calculate the pH of 0.010 molarity acetic acid solution, if its dissociation constant is 1.8*10^-5 - Quora
![What is the pH of a 1 M CH3COOH solution? [ Ka of acetic acid = 1.8 × 10^-5, Kw = 10^-14 mol^2 litre^-2 ] What is the pH of a 1 M CH3COOH solution? [ Ka of acetic acid = 1.8 × 10^-5, Kw = 10^-14 mol^2 litre^-2 ]](https://i.ytimg.com/vi/5MXjDjLyUp4/maxresdefault.jpg)
What is the pH of a 1 M CH3COOH solution? [ Ka of acetic acid = 1.8 × 10^-5, Kw = 10^-14 mol^2 litre^-2 ]


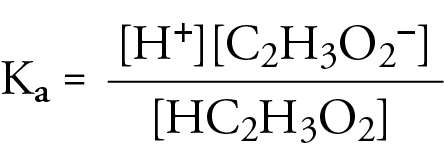

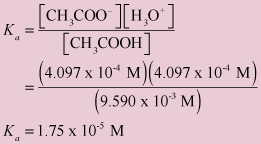

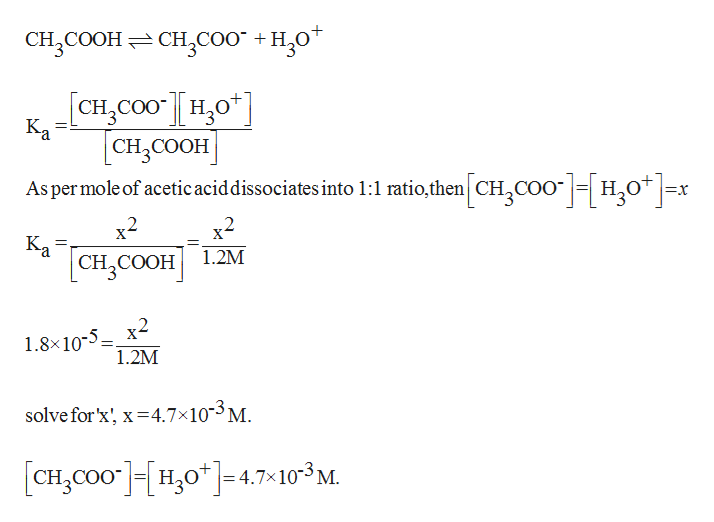
![The pH of 0.01 M solution of acetic acid is 5.0. What are the values of [H^+] and Ka respectively? The pH of 0.01 M solution of acetic acid is 5.0. What are the values of [H^+] and Ka respectively?](https://haygot.s3.amazonaws.com/questions/1445613_1156485_ans_e20f838d2ffe4d0eb21c5b51d09eb783.jpg)