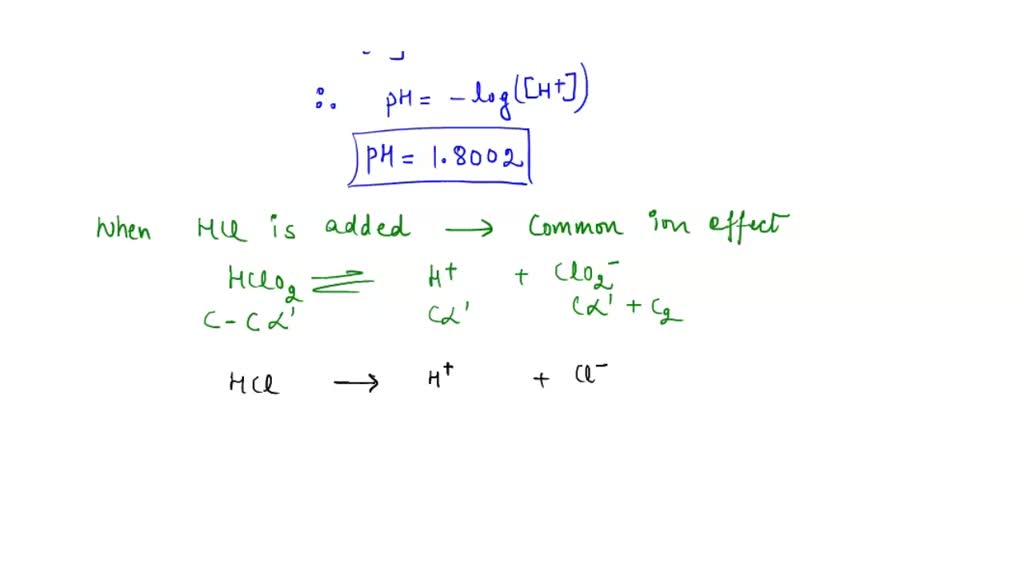Ka for CH3COOH is 1.8×10^-5. find out the % dissociation of 0.2M CH3COOH in 0.1M HCl solution? - EduRev NEET Question

Reaction Kinetics of HCl Catalytic Oxidation over a Supported Cu-Based Composite Industrial Catalyst | Industrial & Engineering Chemistry Research

Ka, Kb. Comparing the pH of two acids 1.Predict the pH of HCl and HF (below) 2.Calibrate a pH meter 3.Measure the pH of HCl(aq) and HF(aq) 4.Complete. - ppt download

Binding Association Constants (Ka ) and Binding Sites (N) for Three... | Download Scientific Diagram