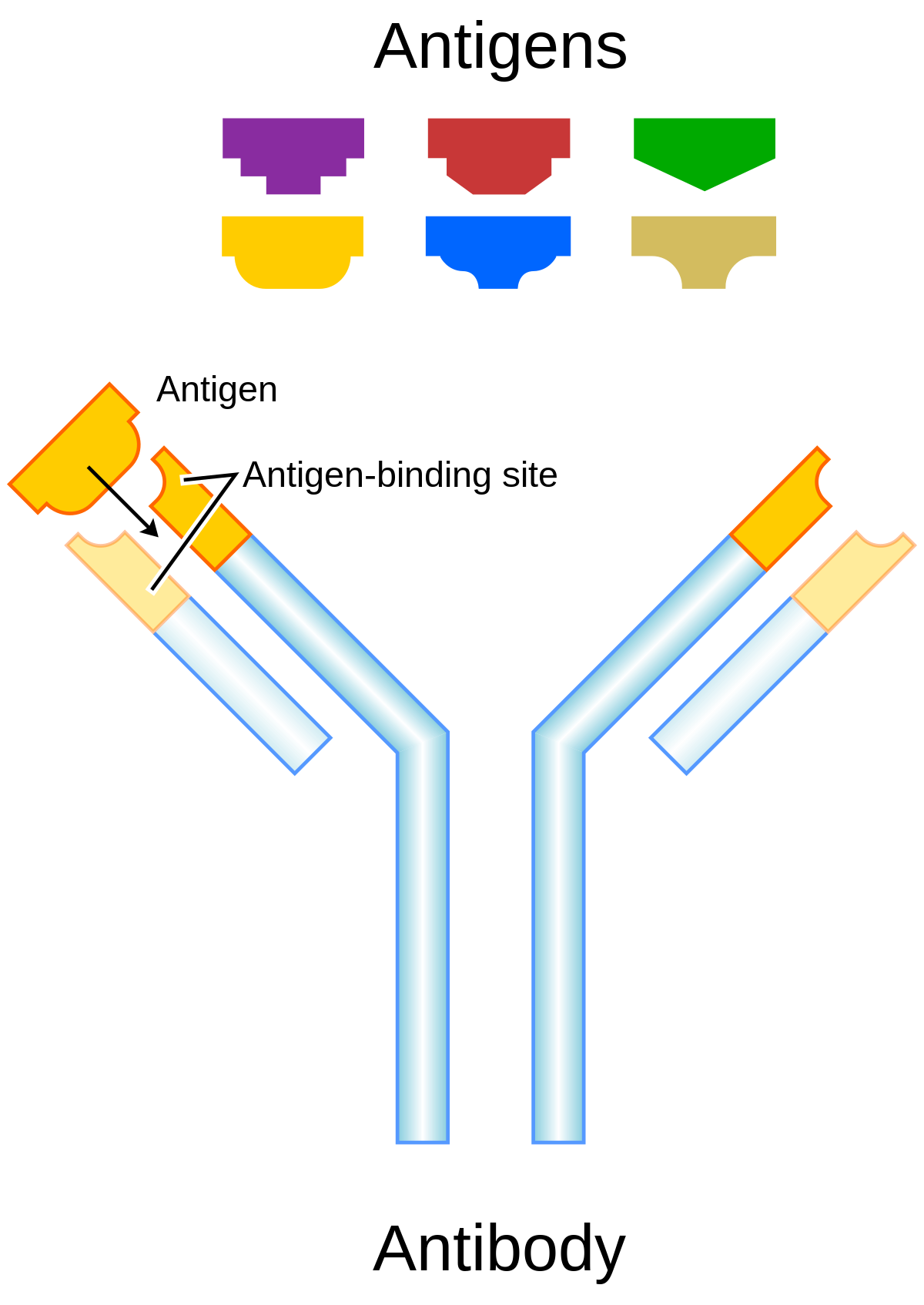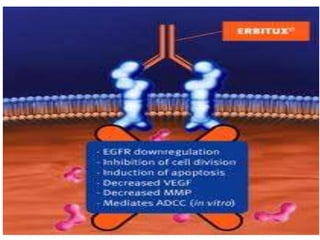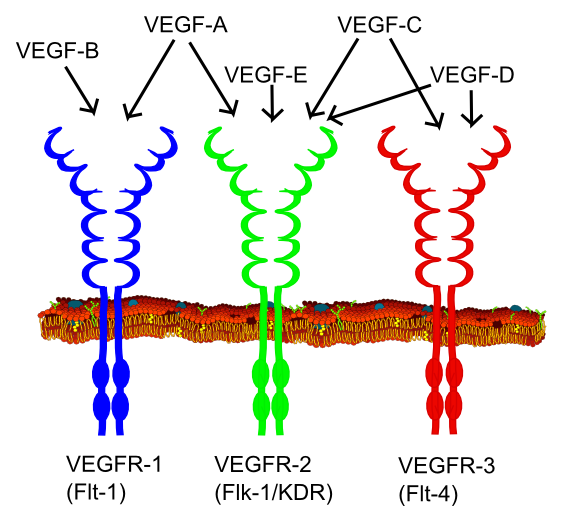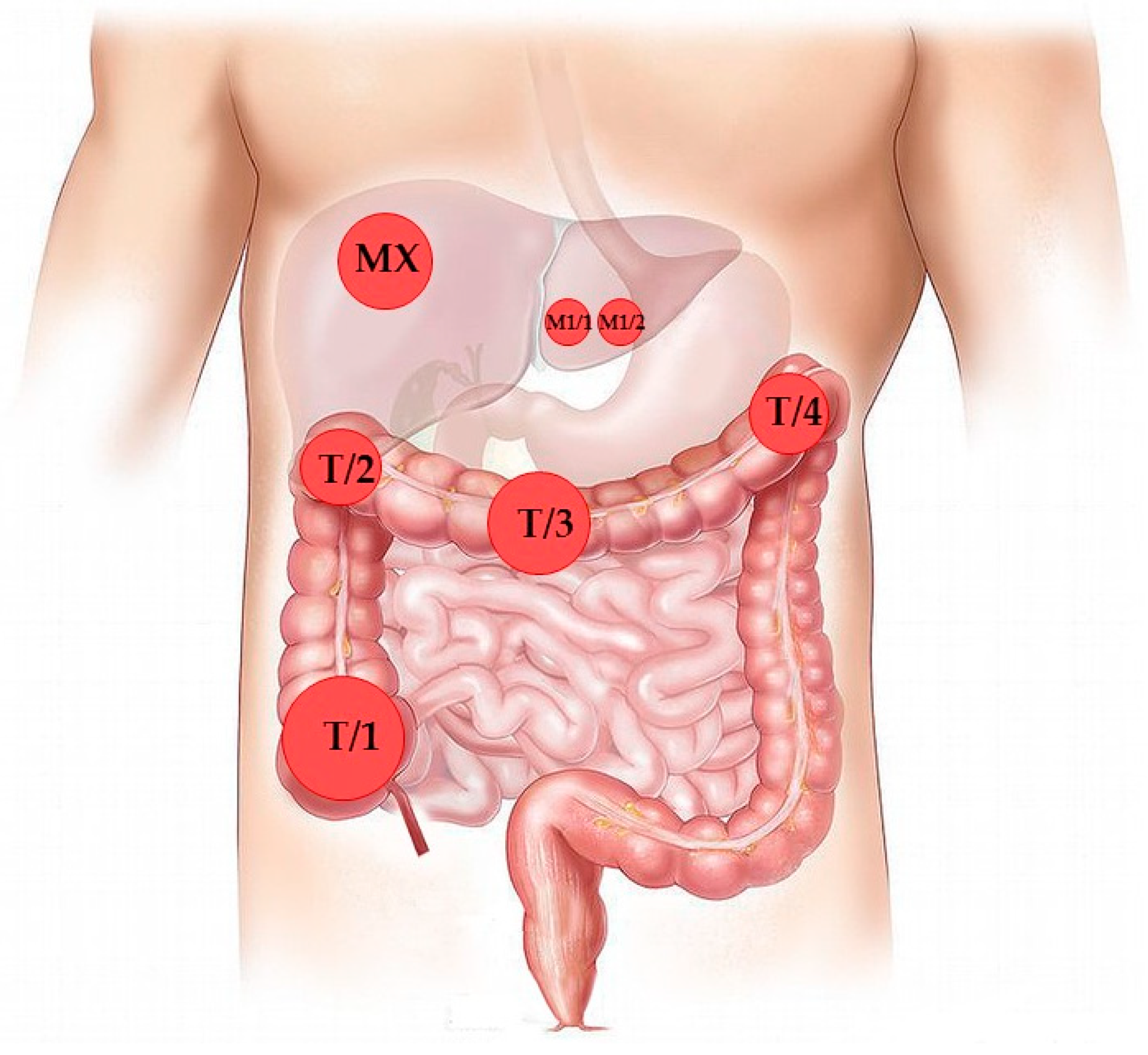
Diagnostics | Free Full-Text | Quadruplicate Synchronous Adenocarcinoma of the Colon with Distant Metastases—Long-Term Molecular Follow-Up by KRAS and TP53 Mutational Profiling

Skin Toxicity Evaluation Protocol With Panitumumab (STEPP), a Phase II, Open-Label, Randomized Trial Evaluating the Impact of a Pre-Emptive Skin Treatment Regimen on Skin Toxicities and Quality of Life in Patients With

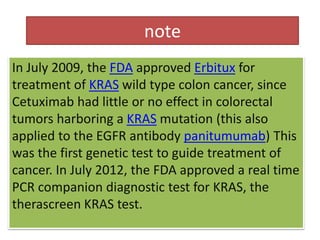
QIAGEN Receives FDA Approval of therascreen® KRAS RGQ PCR Kit Paired with Second Colorectal Cancer Drug

Skin Toxicity Evaluation Protocol With Panitumumab (STEPP), a Phase II, Open-Label, Randomized Trial Evaluating the Impact of a Pre-Emptive Skin Treatment Regimen on Skin Toxicities and Quality of Life in Patients With

Bispecific antibodies for cancer therapy: the light at the end of the tunnel? - Abstract - Europe PMC

Wild-Type KRAS Is Required for Panitumumab Efficacy in Patients With Metastatic Colorectal Cancer | Journal of Clinical Oncology